Structure Prototypes
In materials science, a structure prototype is a representative structure that captures the essential features of a particular crystal structure or atomic arrangement. Structure prototypes are used to describe the symmetry, geometry, and connectivity of atoms in a crystal structure without specifying the exact atomic positions. By defining a structure prototype, researchers can classify and compare different crystal structures based on their underlying symmetry and geometry.
FCC, BCC, and HCP Structures¶
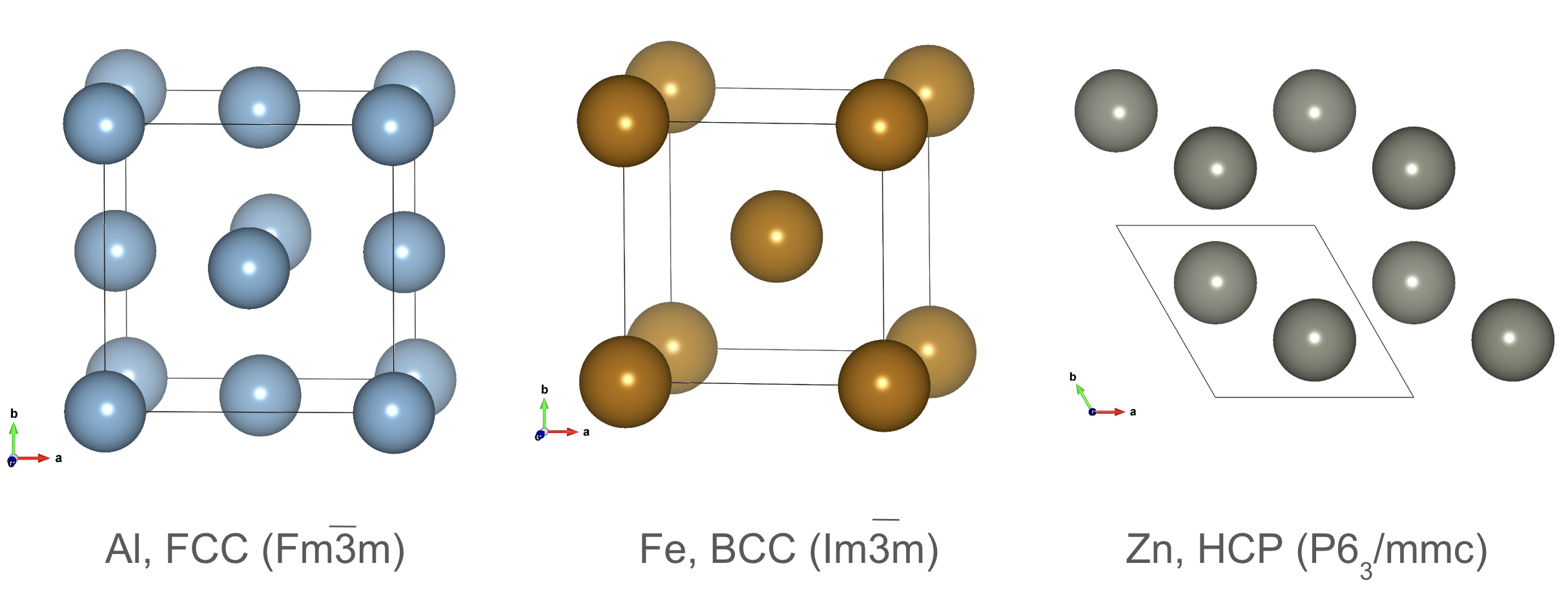
Crystal structure prototypes: FCC, BCC, and HCP.
Three common structure prototypes in materials science are the face-centered cubic (FCC), body-centered cubic (BCC), and hexagonal close-packed (HCP) structures. These prototypes represent the fundamental arrangements of atoms in cubic, hexagonal, and close-packed crystal structures, respectively.
FCC and HPC structures are close-packed structures, with the FCC structure having atoms at the corners and face centers of a cube, while the HCP structure has atoms at the corners and centers of the top and bottom faces of a hexagonal prism. Their packing efficiency is 74%. The BCC structure has atoms at the corners and the center of a cube.
Rocksalt and CsCl Structures¶
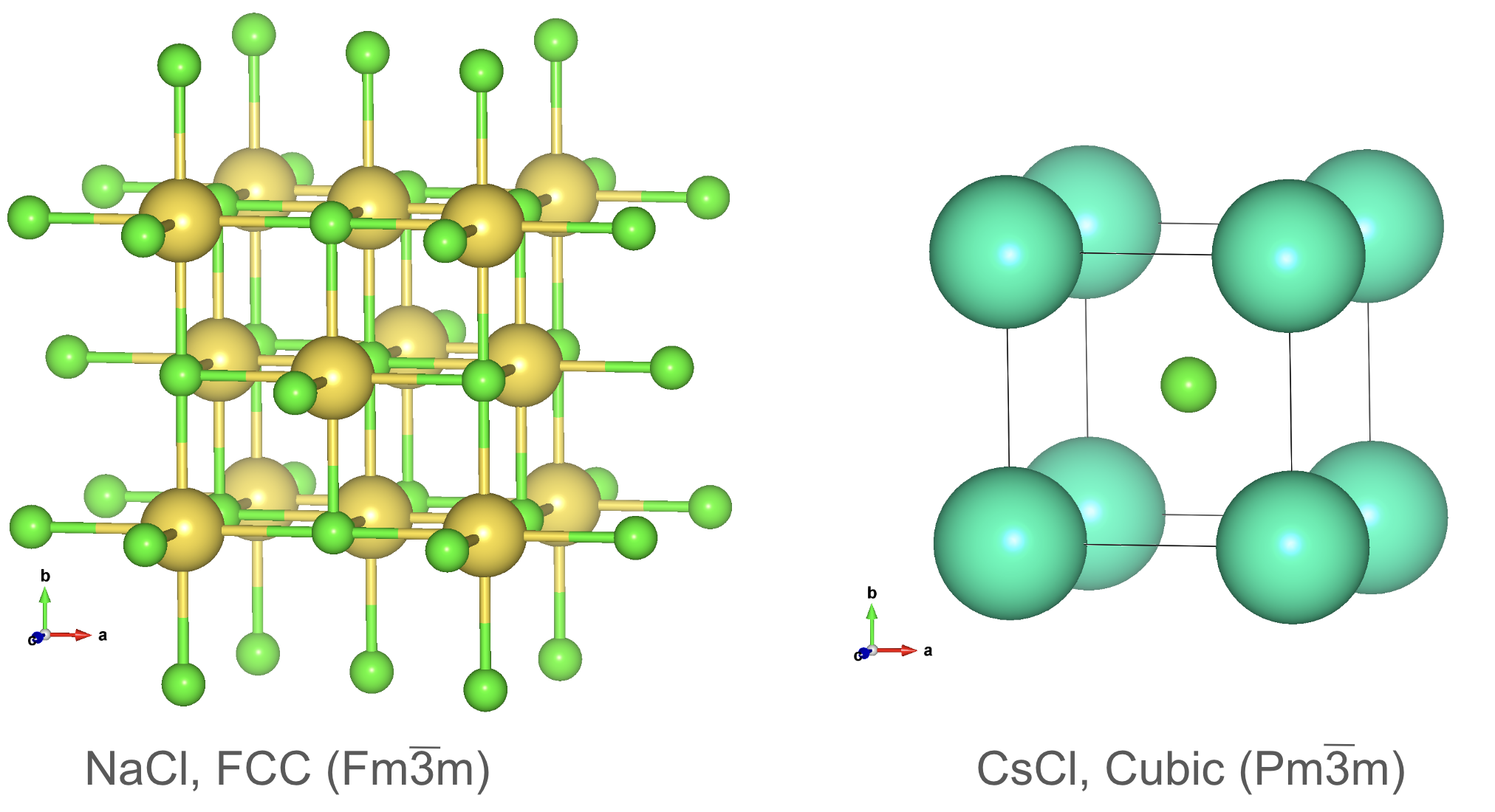
Crystal structure prototypes: Rocksalt and CsCl.
Other structure prototypes include the rocksalt (NaCl) and cesium chloride (CsCl) structures, which are commonly found in ionic compounds. The rocksalt structure consists of alternating cation and anion layers, while the CsCl structure has a simple cubic lattice with a cation at the center and an anion at each corner.
Perovskite Structure¶
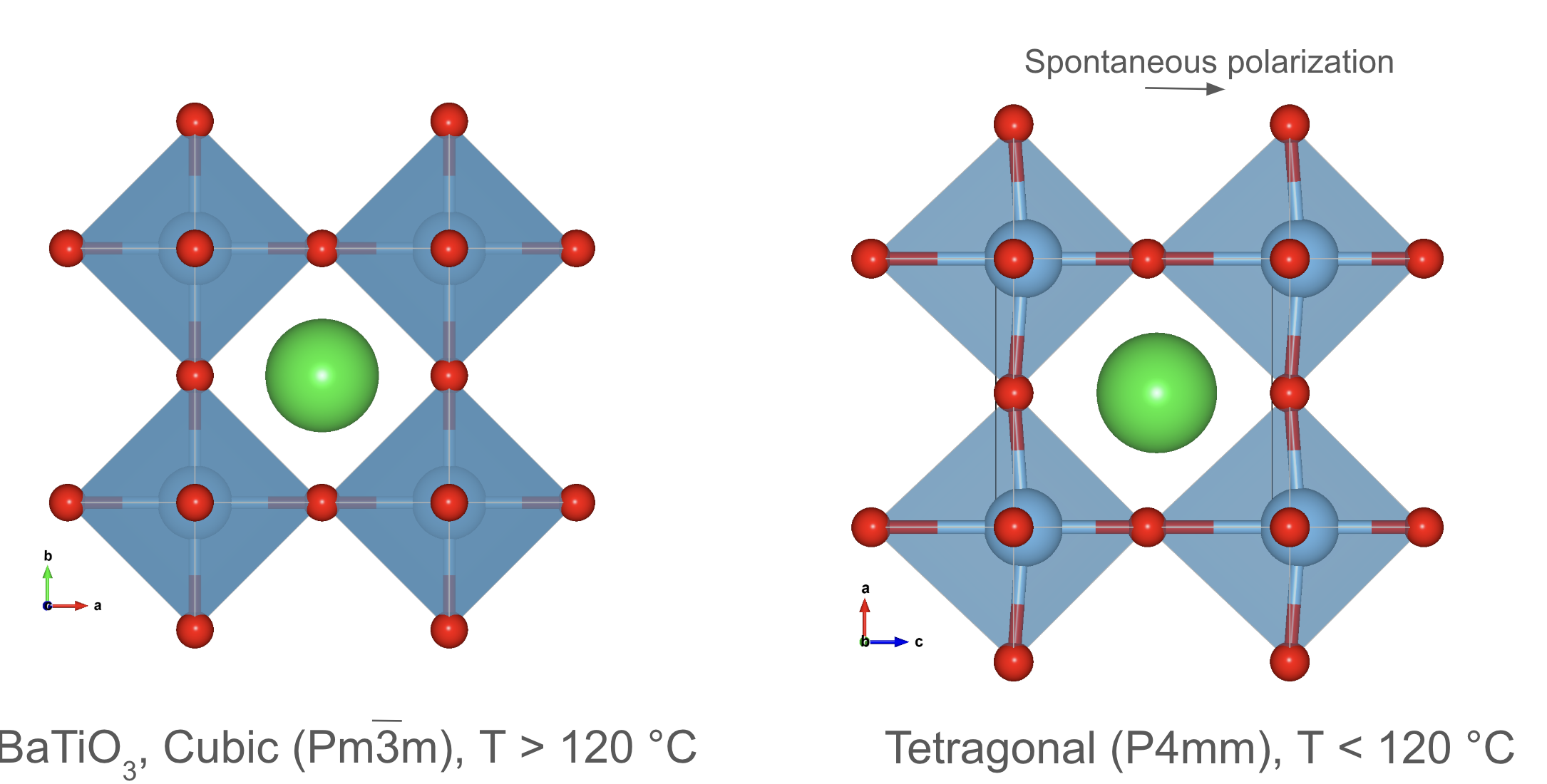
Crystal structure prototypes: Perovskite (). It undergoes a phase transition from a cubic to a tetragonal structure below 120 °C.
The perovskite structure is another important prototype that is widely used in materials science. It consists of a cubic unit cell with a cation at the center, an anion at the corners, and additional cations at the face centers. Perovskite materials exhibit a wide range of properties, including ferroelectricity, magnetism, and superconductivity.
An example is the compound , which is a well-known perovskite material with ferroelectric properties. has a perovskite structure with a octahedral unit surrounded by cations. Below 120 °C, undergoes a phase transition from a cubic to a tetragonal structure, leading to the formation of spontaneous electric dipoles, which give rise to its ferroelectric behavior.
Zincblend and Wurtzite Structures¶
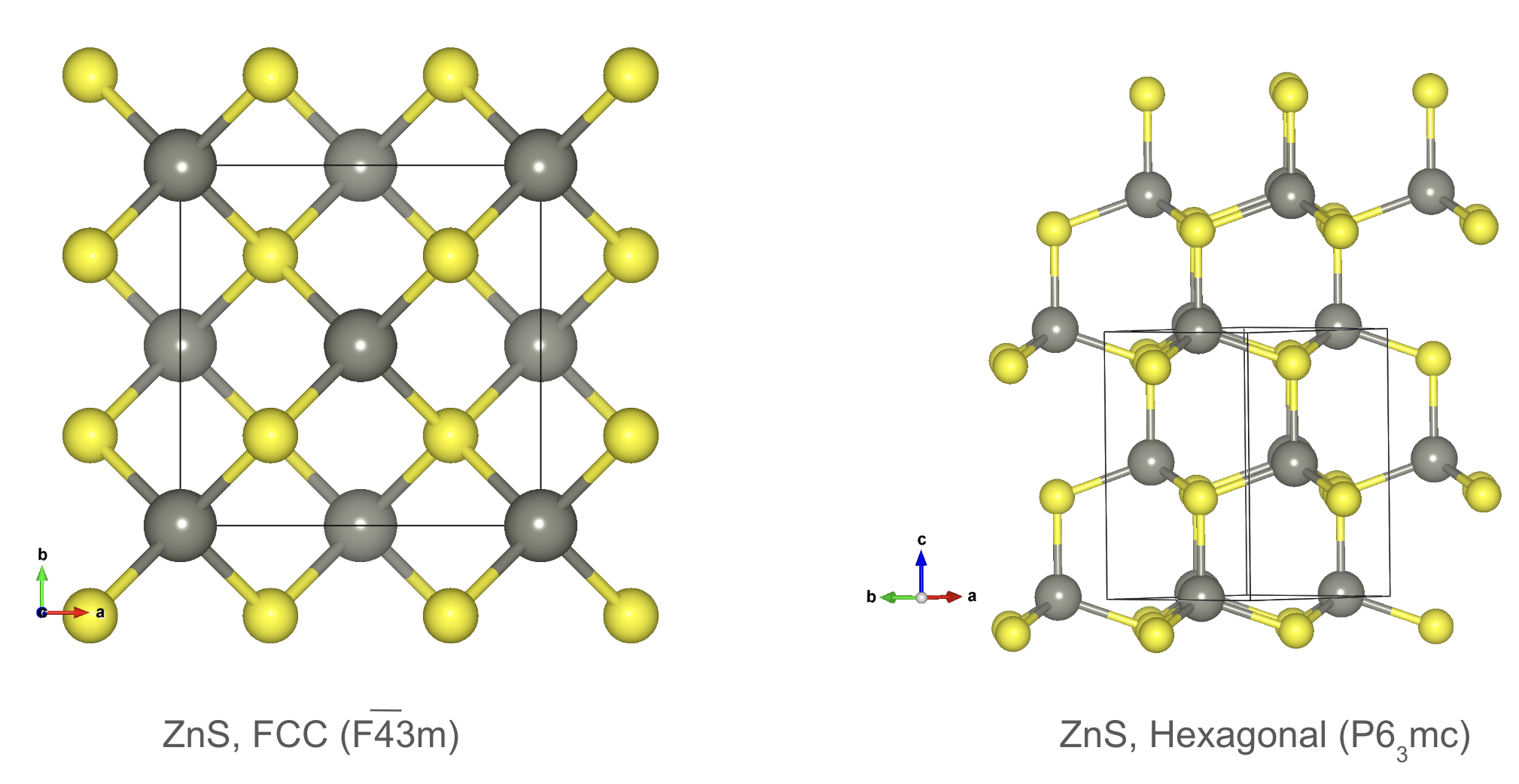
Crystal structure prototypes: Zincblende and Wurtzite.
The zincblende and wurtzite structures are common prototypes for binary compounds such as and . The zincblende structure consists of two interpenetrating face-centered cubic lattices, while the wurtzite structure has a hexagonal close-packed arrangement with alternating cations and anions along the c-axis.
Garnet, Spinel, and Rutile Structures¶
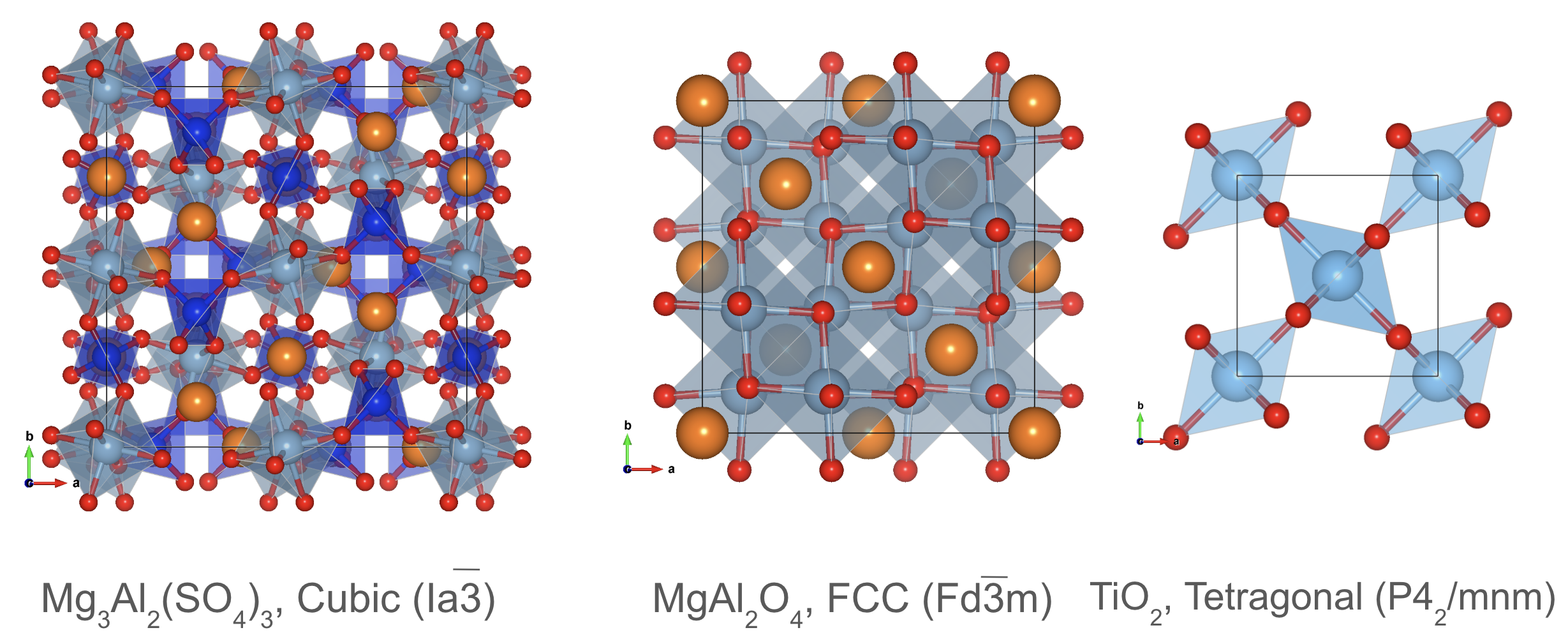
Crystal structure prototypes: Garnet, Spinel, and Rutile.
Other structure prototypes include the garnet, spinel, and rutile structures, which are found in a variety of materials. The garnet structure is characterized by a cubic unit cell with cations at the corners and anions at the centers of the faces. The spinel structure consists of two interpenetrating face-centered cubic lattices with cations at the tetrahedral and octahedral sites. The rutile structure has a tetragonal unit cell with cations at the corners and oxygen atoms at the centers of the edges.